医薬品製造における充填・滅菌など各種包装機の世界シェア最大級のメーカーであるシンテゴン社の日本法人にて、医薬品の自動検査ライン向け自社製品 (自動検査機)の製造・調整・検査といったエンジニアリング業務を担当いただきます。
・医薬品向け自動検査機械の製造、調整、検査業務全般
・医薬品向け自動検査機械のフィールドサービス業務(客先での部品交換、トラブル対応)
・受注プロジェクトにおける機械の据え付け業務(組み立て調整、客先での搬入時の通線、結線、電気的社内検証作業、客先での検証作業、電気的トラブル対応のサポート)
・既設機械の改造工事や移設作業に伴う機械調整業務
・バリテーション(据付時適格性検査)の補助業務
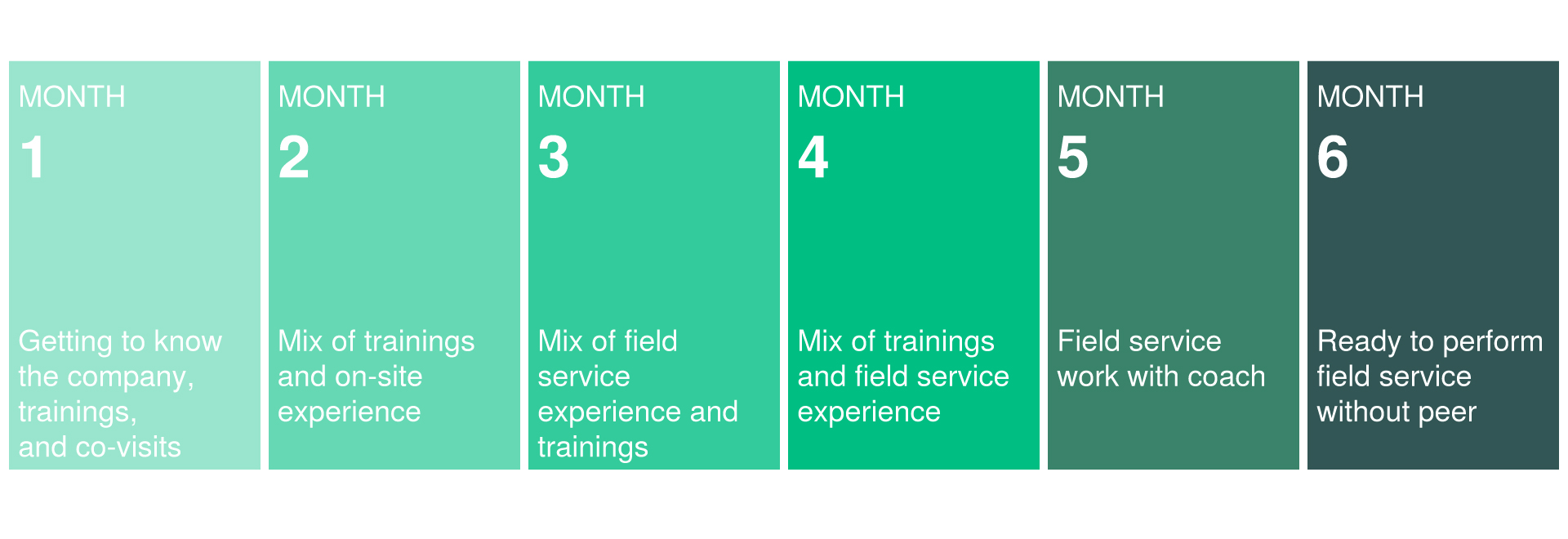
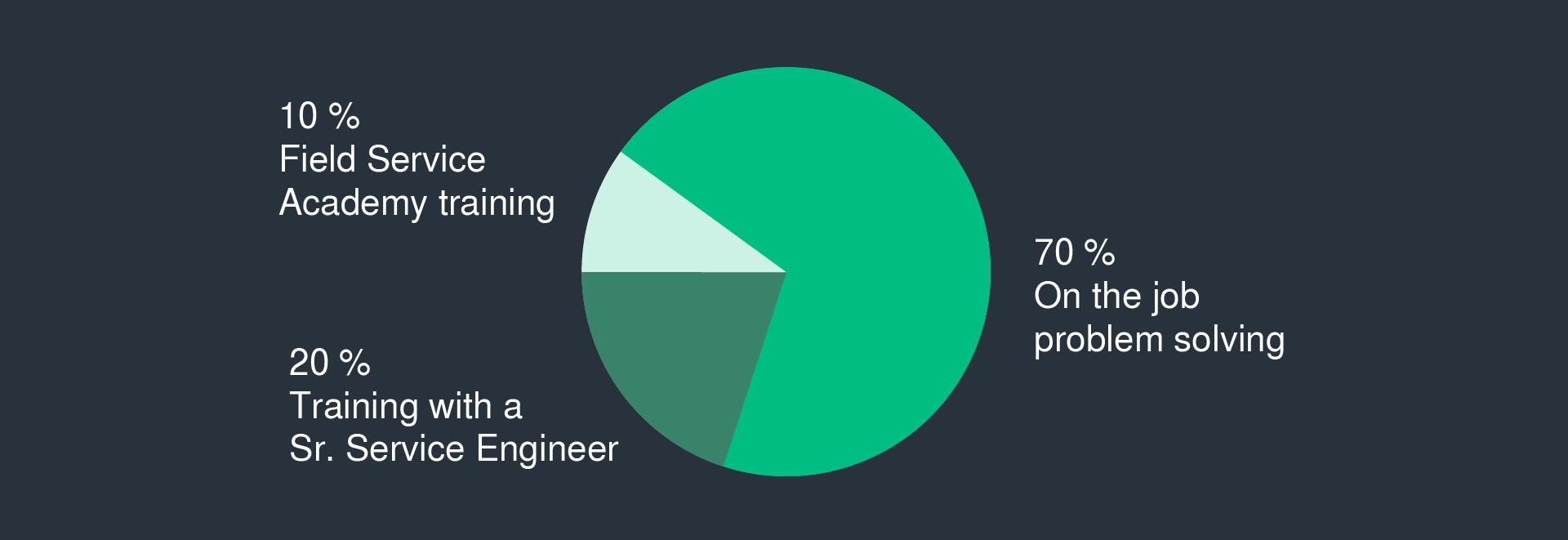
担当製品は国内製のため、自社工場内での作業(機械調整、テスト)と、客先での出張作業(据え付け、試運転)の組み合わせとなります。国内顧客向けであれば国内出張、海外顧客向けであれば海外出張での作業となります。期間は案件の規模によって異なりますが、2週間~2、3か月程度となります。
This position is responsible for engineering work such as manufacturing, adjustment, and inspection of our company's products (automatic inspection machines) for automatic inspection lines for pharmaceuticals at the Japanese subsidiary of Syntegon, one of the world's largest manufacturers of various packaging machines for filling and sterilization in pharmaceutical manufacturing.
-General manufacturing, adjustment, and inspection work for automatic inspection machines for pharmaceuticals
-Field service work for automatic inspection machines for pharmaceuticals (part replacement at customer's site, troubleshooting)
-Machine installation work for order projects (assembly adjustment, wiring at customer's site when delivered, wiring, electrical in-house verification work, verification work at customer's site, support for electrical trouble response)
-Machine adjustment work associated with modification work and relocation work of existing machines
-Support work for validation (qualification test at installation)
Since the products you are in charge of are manufactured domestically, you will be combined with work in our own factory (machine adjustment, testing) and on-site work at customer's site (installation, trial operation). For domestic customers, you will be on-site in Japan, and for overseas customers, you will be on-site overseas. The period will vary depending on the scale of the project, but it will be around 2 weeks to 2-3 months.